
Not actual patient.
PEDMARK reduced the risk of permanent damage from cisplatin-induced ototoxicity1

Not actual patient.
PEDMARK was specifically developed for pediatric use
- Evaluated in randomized clinical trials in patients 1 month of age and older
- Product and administration protocol were approved together to ensure efficacious and safe delivery of STS for patients with solid tumors
There is no substitute for FDA approval
- PEDMARK dosing, including dose modifications for younger and lower-weight patients, are specific to the PEDMARK formulation
- Off-label use of compounded STS raises potential safety issues due to acute risk associated with intravenous potassium chloride excipient in patients
| KCI Concentration (mEq/L) | Rate of KCI Infusion (mEq/kg/h) | |||||
|---|---|---|---|---|---|---|
| PEDMARK1 | LRS2+KCIa | Sodium Thiosulfate (STS) Product3,b | PEDMARK1 | LRS2+KCIa | STS Product3,b | |
| Infusion for 1-mo-old (BSA=0.25 m²), weight=4.2 kg | 0 | 14 | 30 | 0 | 0.06 | 0.6 |
| Infusion for 2-yr-old (BSA=0.54 m²), weight=12.8 kg | 24 | 0.09 | 0.8 | |||
a=When administering over 15 minutes according to the PEDMARK label; b=When administering according to the CHOP treatment protocol.
There is no substitute for PEDMARK
| Formulation Component | PEDMARK1 | STS from Hope Pharmaceuticals |
|---|---|---|
| FDA-approved STS formulation for cisplatin-induced ototoxicity | Yes | No |
| Unique formulation of STS specifically developed for pediatric use | Yes | No |
| Clinically tested in children in two phase 3 randomized trials that took a decade to recruit | Yes | No |
| Potassium chloride has been removed and the levels of boron have been lowered | Yes | No |
| Prediluted and ready-to-administer | Yes | No |
| Can be safely administered multiple times as required by the chemotherapy treatment protocol | Yes | No |
There is only one PEDMARK
There is only one PEDMARK
While it is not fully understood how STS reduces the risk of ototoxicity, the mechanism of action may include1:
- Increasing levels of endogenous antioxidants
- Scavenging reactive oxygen species
- Direct interaction between cisplatin and STS
STS can enter cells at least partly through the sodium sulfate cotransporter 2 and can cause intracellular effects such as1:
- Increase in antioxidant glutathione levels
- Inhibition of intracellular oxidative stress
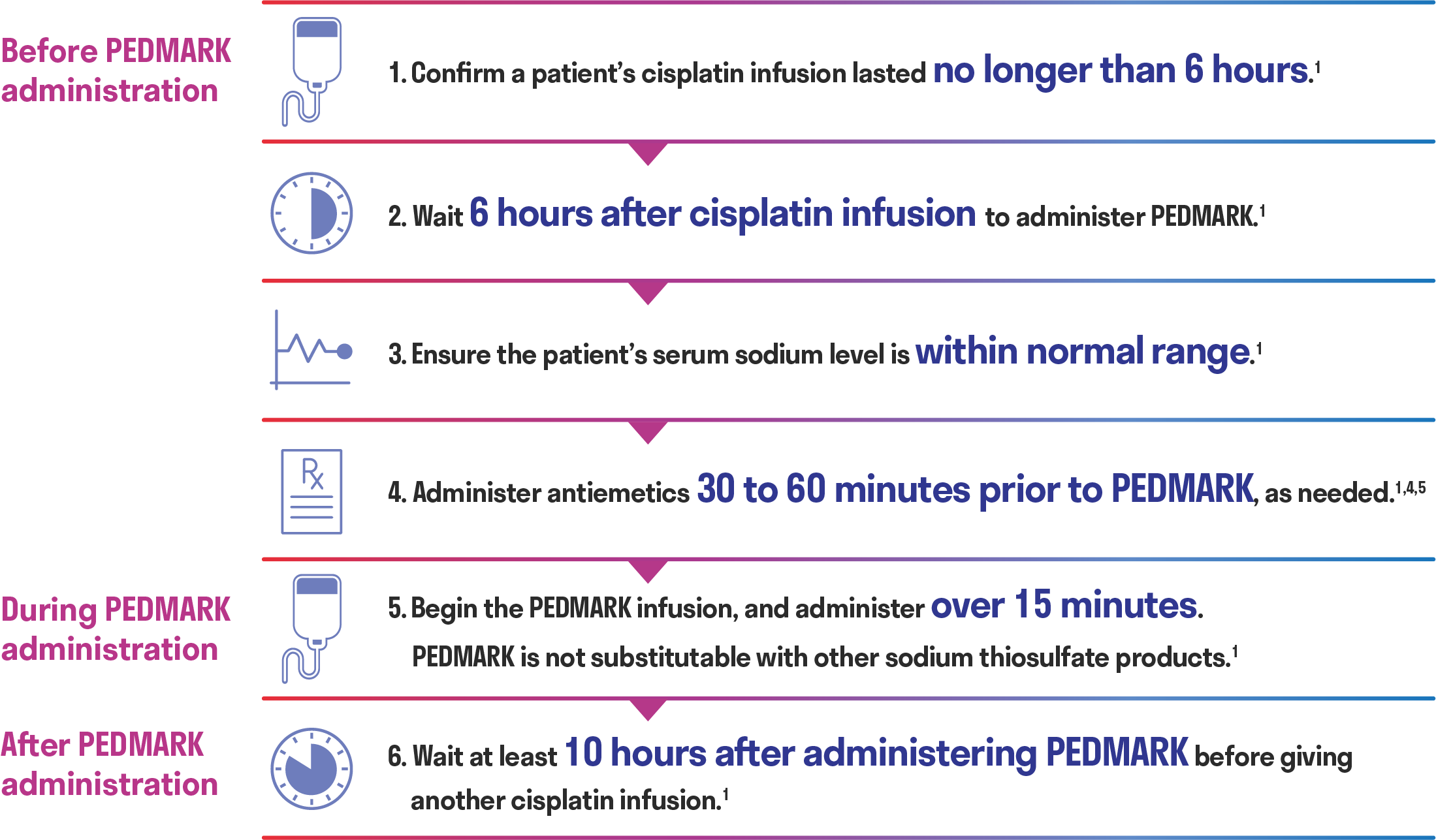
Proven administration protocol
There are timing considerations when administering PEDMARK to minimize potential interference with cisplatin antitumor activity.1
- Unbound cisplatin has a half-life of about 0.6 to 1.5 hours and is below the limit of detection in the bloodstream 3 hours post-infusion6,7
- STS does not readily cross cell membranes and distributes mainly extracellularly, without binding to human plasma proteins1
- This distribution, when coupled with the 6-hour wait post-cisplatin infusion for administering PEDMARK, should prevent a tumor protective effect of STS1
- It is unlikely that significant bioactive levels of cisplatin are present 6 hours post-infusion2,8
- PEDMARK infusions must be completed at least 10 hours before the next cisplatin infusion1
Wait at least 10 hours after administering PEDMARK before administering another round of cisplatin1
- Administer antiemetics 30 to 60 minutes prior to PEDMARK, as needed1,5
- For patients who experience a hypersensitivity reaction, administer antihistamines and glucocorticoids (if appropriate) before each subsequent PEDMARK infusion1
Helpful Tools
Resources for patients and caregivers
IMPORTANT SAFETY INFORMATION
- PEDMARK is contraindicated in patients with history of a severe hypersensitivity to sodium thiosulfate or any of its components.
- Hypersensitivity reactions occurred in 8% to 13% of patients in clinical trials. Monitor patients for hypersensitivity reactions. Immediately discontinue PEDMARK and institute appropriate care if a hypersensitivity reaction occurs. Administer antihistamines or glucocorticoids (if appropriate) before each subsequent administration of PEDMARK. PEDMARK may contain sodium sulfite; patients with sulfite sensitivity may have hypersensitivity reactions, including anaphylactic symptoms and life-threatening or severe asthma episodes. Sulfite sensitivity is seen more frequently in people with asthma.
- PEDMARK is not indicated for use in pediatric patients less than 1 month of age due to the increased risk of hypernatremia or in pediatric patients with metastatic cancers.
- Hypernatremia occurred in 12% to 26% of patients in clinical trials, including a single Grade 3 case. Hypokalemia occurred in 15% to 27% of patients in clinical trials, with Grade 3 or 4 occurring in 9% to 27% of patients. Monitor serum sodium and potassium at baseline and as clinically indicated. Withhold PEDMARK in patients with baseline serum sodium greater than 145 mmol/L.
- Monitor for signs and symptoms of hypernatremia and hypokalemia more closely if the glomerular filtration rate (GFR) falls below 60 mL/min/1.73 m2.
- Administer antiemetics prior to each PEDMARK administration. Provide additional antiemetics and supportive care as appropriate.
- The most common adverse reactions (≥25% with difference between arms of >5% compared to cisplatin alone) in SIOPEL 6 were vomiting, nausea, decreased hemoglobin, and hypernatremia. The most common adverse reaction (≥25% with difference between arms of >5% compared to cisplatin alone) in COG ACCL0431 was hypokalemia.
Please see full Prescribing Information for PEDMARK.
INDICATIONS AND USAGE
PEDMARK (sodium thiosulfate injection) is indicated to reduce the risk of ototoxicity associated with cisplatin in pediatric patients 1 month of age and older with localized, non‑metastatic solid tumors.
Limitations of Use
The safety and efficacy of PEDMARK have not been established when administered following cisplatin infusions longer than 6 hours. PEDMARK may not reduce the risk of ototoxicity when administered following longer cisplatin infusions, because irreversible ototoxicity may have already occurred.

