
Not actual patient.
PEDMARK is administered as a 15‑minute infusion1

Not actual patient.
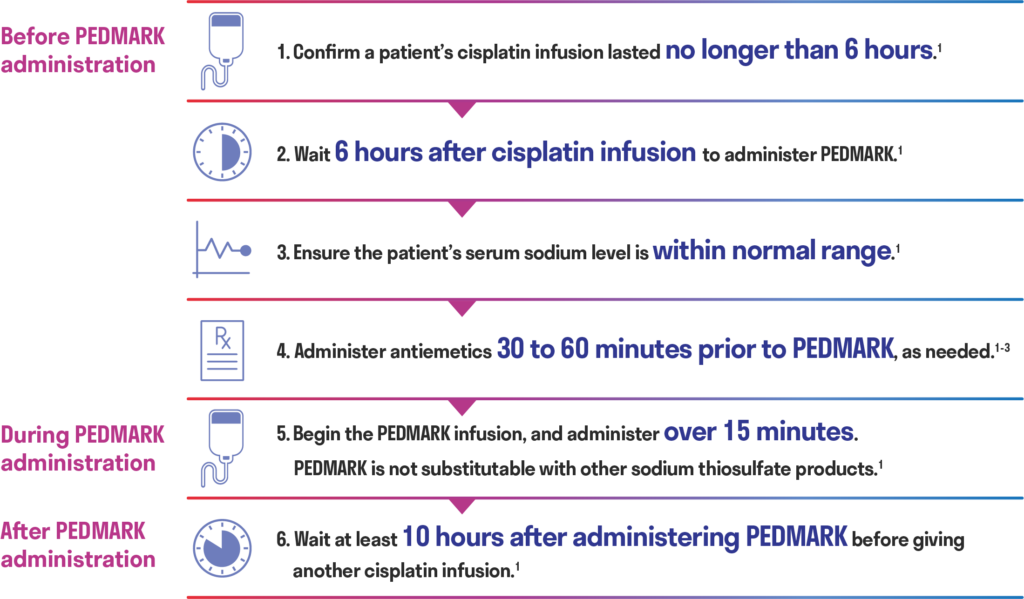
Administering PEDMARK1
Remember to plan for PEDMARK
- Patient’s cisplatin infusion must last no longer than 6 hours1
- Wait 6 hours after cisplatin infusion to administer PEDMARK1
- Wait at least 10 hours after administering PEDMARK before giving another cisplatin infusion1
Recommended for patients who will receive emetogenic anticancer agents by the National Comprehensive Cancer Network® (NCCN®)
These recommendations are not consistent with the FDA indication. Always refer to the PEDMARK® (sodium thiosulfate injection) Prescribing Information and Instructions for Use.

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis recommends a prophylactic antiemetic regimen for patients receiving emetogenic anticancer agents (per institutional/clinician preferences).4
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network.
Dosing for PEDMARK is based upon each patient’s surface area according to actual body weight1
| Body Weight | PEDMARK Dose |
|---|---|
| Less than 5 kg | 10 g/m2 |
| 5 to 10 kg | 15 g/m2 |
| Greater than 10 kg | 20 g/m2 |
Recommended premedications
Recommended dose modifications for
adverse reactions1
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| Hypersensitivity | Grade 3 or higher | Permanently discontinue PEDMARK. |
| Hypernatremia | >145 mmol/L | Withhold next dose until levels return to within normal limits. Resume at the same dose. |
| Hypokalemia | Grade 3 or 4 | Withhold next dose until levels return to within normal limits. Resume at the same dose. |
| Other adverse reactions | Grade 3 | Withhold until ≤Grade 1. Resume at the same dose. |
| Grade 4 | Permanently discontinue PEDMARK. |
Recommended premedications
Administer antiemetics 30 to 60 minutes prior to PEDMARK, as needed.1-3
For patients who experience a hypersensitivity reaction, administer antihistamines and glucocorticoids (if appropriate) before each subsequent PEDMARK infusion.
- PEDMARK is available in a single-use vial containing 12.5 g/100 mL (125 mg/mL)
- PEDMARK is not substitutable with other sodium thiosulfate products
Helpful Tools
Resources for patients and caregivers
IMPORTANT SAFETY INFORMATION
- PEDMARK is contraindicated in patients with history of a severe hypersensitivity to sodium thiosulfate or any of its components.
- Hypersensitivity reactions occurred in 8% to 13% of patients in clinical trials. Monitor patients for hypersensitivity reactions. Immediately discontinue PEDMARK and institute appropriate care if a hypersensitivity reaction occurs. Administer antihistamines or glucocorticoids (if appropriate) before each subsequent administration of PEDMARK. PEDMARK may contain sodium sulfite; patients with sulfite sensitivity may have hypersensitivity reactions, including anaphylactic symptoms and life-threatening or severe asthma episodes. Sulfite sensitivity is seen more frequently in people with asthma.
- PEDMARK is not indicated for use in pediatric patients less than 1 month of age due to the increased risk of hypernatremia or in pediatric patients with metastatic cancers.
- Hypernatremia occurred in 12% to 26% of patients in clinical trials, including a single Grade 3 case. Hypokalemia occurred in 15% to 27% of patients in clinical trials, with Grade 3 or 4 occurring in 9% to 27% of patients. Monitor serum sodium and potassium at baseline and as clinically indicated. Withhold PEDMARK in patients with baseline serum sodium greater than 145 mmol/L.
- Monitor for signs and symptoms of hypernatremia and hypokalemia more closely if the glomerular filtration rate (GFR) falls below 60 mL/min/1.73 m2.
- Administer antiemetics prior to each PEDMARK administration. Provide additional antiemetics and supportive care as appropriate.
- The most common adverse reactions (≥25% with difference between arms of >5% compared to cisplatin alone) in SIOPEL 6 were vomiting, nausea, decreased hemoglobin, and hypernatremia. The most common adverse reaction (≥25% with difference between arms of >5% compared to cisplatin alone) in COG ACCL0431 was hypokalemia.
Please see full Prescribing Information for PEDMARK.
INDICATIONS AND USAGE
PEDMARK (sodium thiosulfate injection) is indicated to reduce the risk of ototoxicity associated with cisplatin in pediatric patients 1 month of age and older with localized, non‑metastatic solid tumors.
Limitations of Use
The safety and efficacy of PEDMARK have not been established when administered following cisplatin infusions longer than 6 hours. PEDMARK may not reduce the risk of ototoxicity when administered following longer cisplatin infusions, because irreversible ototoxicity may have already occurred.

