
Not actual patient.
The approval of PEDMARK was based on data from 2 multicenter phase 3 pivotal clinical trials1

Not actual patient.
Not actual patient.
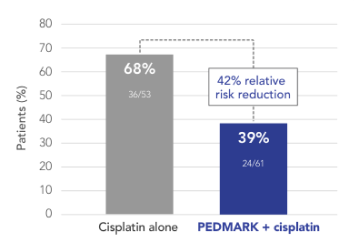
International Childhood Liver Tumor Strategy Group Study (SIOPEL 6)
International Childhood Liver Tumor Strategy Group Study (SIOPEL 6)
Patients 1 month of age and older receiving cisplatin chemotherapy for the treatment of histologically confirmed, newly diagnosed standard risk hepatoblastoma1
Percentage of patients with hearing loss
(ITT population) in SIOPEL 6 (n=114)
Children’s Oncology Group Study
(COG ACCL0431)
Percentage of patients with hearing loss (ITT population) in COG ACCL0431 (n=77)
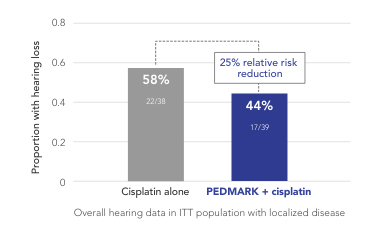
Children’s Oncology Group Study
(COG ACCL0431)
Patients 1 month of age and older receiving cisplatin chemotherapy for the treatment of newly diagnosed germ cell tumor, hepatoblastoma, medulloblastoma, neuroblastoma, osteosarcoma, or any other malignancy treated with cisplatin1
Helpful Tools
Resources for patients and caregivers
IMPORTANT SAFETY INFORMATION
- PEDMARK is contraindicated in patients with history of a severe hypersensitivity to sodium thiosulfate or any of its components.
- Hypersensitivity reactions occurred in 8% to 13% of patients in clinical trials. Monitor patients for hypersensitivity reactions. Immediately discontinue PEDMARK and institute appropriate care if a hypersensitivity reaction occurs. Administer antihistamines or glucocorticoids (if appropriate) before each subsequent administration of PEDMARK. PEDMARK may contain sodium sulfite; patients with sulfite sensitivity may have hypersensitivity reactions, including anaphylactic symptoms and life-threatening or severe asthma episodes. Sulfite sensitivity is seen more frequently in people with asthma.
- PEDMARK is not indicated for use in pediatric patients less than 1 month of age due to the increased risk of hypernatremia or in pediatric patients with metastatic cancers.
- Hypernatremia occurred in 12% to 26% of patients in clinical trials, including a single Grade 3 case. Hypokalemia occurred in 15% to 27% of patients in clinical trials, with Grade 3 or 4 occurring in 9% to 27% of patients. Monitor serum sodium and potassium at baseline and as clinically indicated. Withhold PEDMARK in patients with baseline serum sodium greater than 145 mmol/L.
- Monitor for signs and symptoms of hypernatremia and hypokalemia more closely if the glomerular filtration rate (GFR) falls below 60 mL/min/1.73 m2.
- Administer antiemetics prior to each PEDMARK administration. Provide additional antiemetics and supportive care as appropriate.
- The most common adverse reactions (≥25% with difference between arms of >5% compared to cisplatin alone) in SIOPEL 6 were vomiting, nausea, decreased hemoglobin, and hypernatremia. The most common adverse reaction (≥25% with difference between arms of >5% compared to cisplatin alone) in COG ACCL0431 was hypokalemia.
Please see full Prescribing Information for PEDMARK.
INDICATIONS AND USAGE
PEDMARK (sodium thiosulfate injection) is indicated to reduce the risk of ototoxicity associated with cisplatin in pediatric patients 1 month of age and older with localized, non‑metastatic solid tumors.
Limitations of Use
The safety and efficacy of PEDMARK have not been established when administered following cisplatin infusions longer than 6 hours. PEDMARK may not reduce the risk of ototoxicity when administered following longer cisplatin infusions, because irreversible ototoxicity may have already occurred.

