Not actual patient.
Cisplatin-induced ototoxicity puts many cancer patients at risk for lifelong hearing impairment1

Not actual patient.
Not actual patient.
Understanding how cisplatin-induced ototoxicity occurs
The bones of the inner ear ossify around 23 weeks’ gestation and are then at its adult size and form. A fetus can hear from around 26 weeks’ gestation.2
Cisplatin-induced ototoxicity is characterized by the production of toxic levels of reactive oxygen species within the cochlea, resulting in the destruction of cochlear hair cells and damage to the stria vascularis and spiral ganglion cells.3
Once cochlear hair cells are destroyed, they cannot grow back. This damage is generally dose-dependent, bilateral, and irreversible.3 Patients may begin to experience symptoms like tinnitus and vertigo.4 The result may be lifelong hearing loss that will affect almost every aspect of a patient’s life.3
Risk of cisplatin-induced hearing loss has been shown to be substantially higher among patients who are younger than 5 years of age when undergoing chemotherapy.5

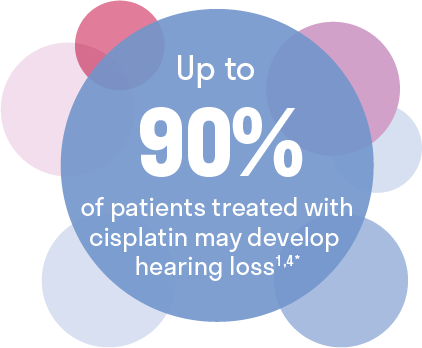
The majority of cancer patients will develop ototoxicity
- ~5000 children (≤18 years) are diagnosed with a solid tumor and treated with platinum-based chemotherapy such as cisplatin every year in the United States6,7
- ~70% (3500) of these patients are diagnosed with localized, non-metastatic disease8
Cisplatin carries the highest risk of ototoxicity among all FDA-approved platinum compounds1
Just 1 cisplatin treatment cycle can lead to a lifetime of hearing loss1
From the very first treatment cycle, patients can suffer cisplatin-induced ototoxicity that may progressively worsen even after treatment ends1
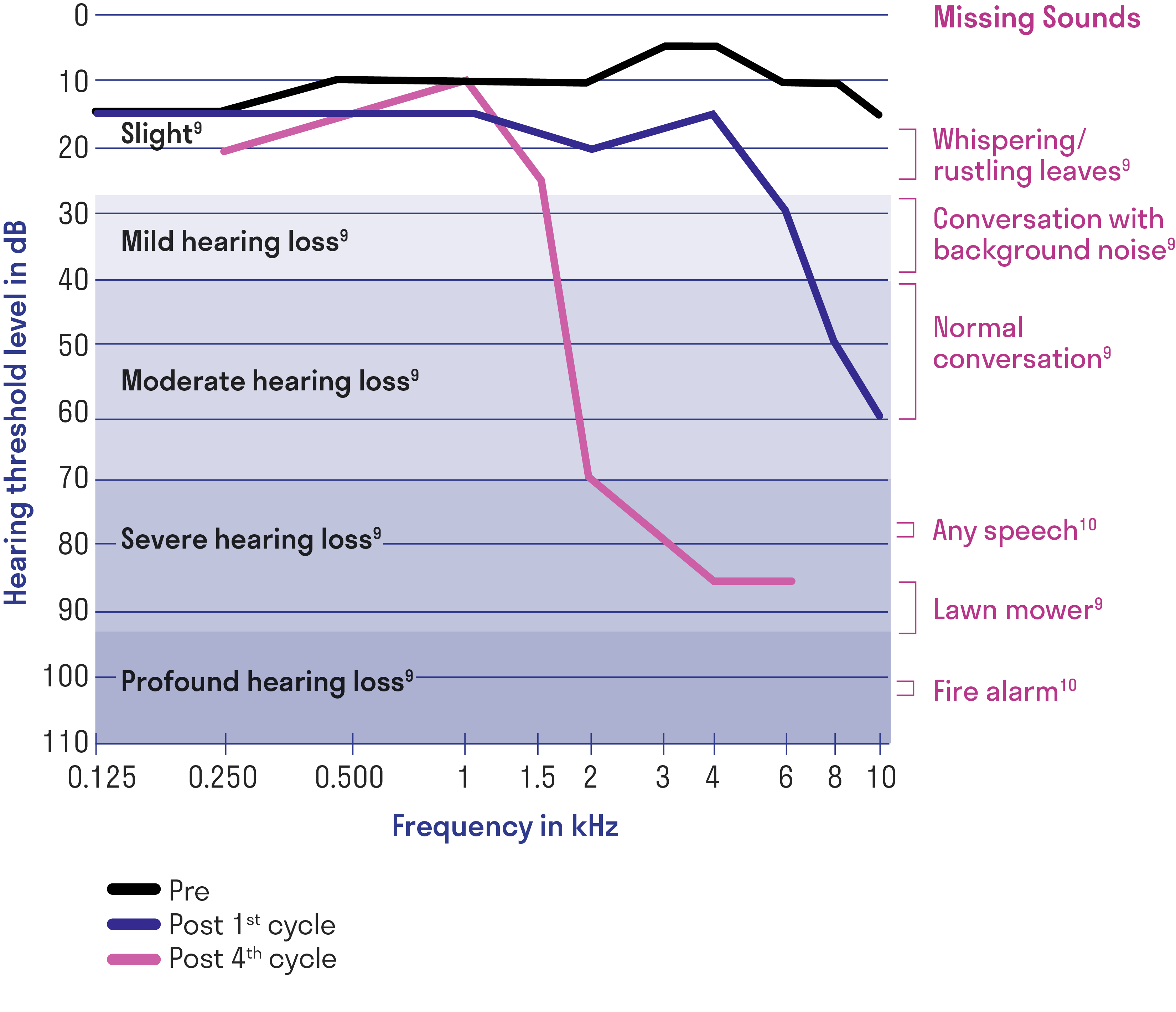
Progressive hearing loss during cisplatin therapy1
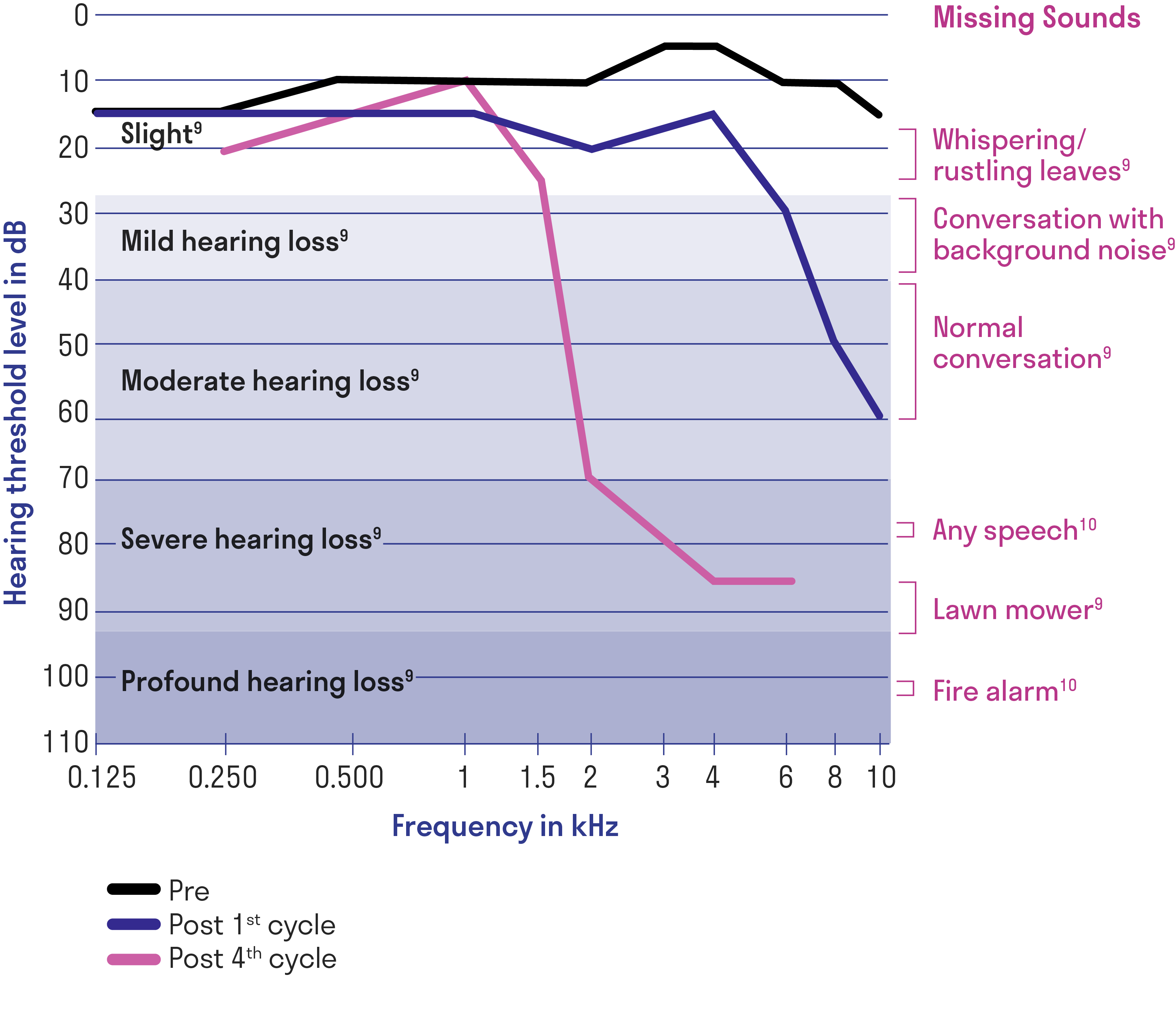
Left ear audiogram of a single patient at baseline before cisplatin treatment, after the first cycle, and after 4 cycles of cisplatin.
Data may not be representative of all patients.
Adapted from Langer T, et al.
Progressive and irreversible hearing loss can have a profound
lifelong impact on patients11-13
The overall 5-year survival rate for patients with localized, non-metastatic disease is 80% to 90%, making the permanent and progressive impact of ototoxicity an important consideration.11,14
The burden of hearing loss in survivors exerts a toll on many crucial aspects of their lives, such as:

Speech and language skills12

Social-emotional development12,13
Career potential13

Ability to live independently13

Academic performance12,13
Increased monitoring is recommended when caring for
cancer patients12
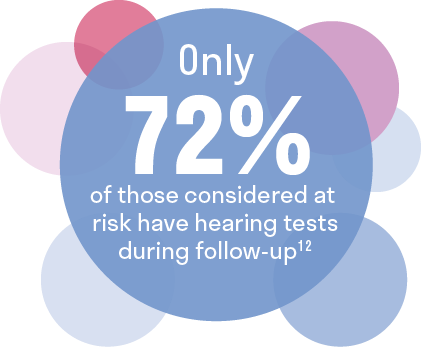
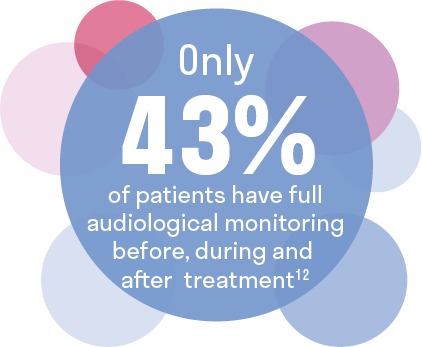
Audiologic monitoring is underutilized in cancer patients
Helpful Tools
Resources for patients and caregivers
IMPORTANT SAFETY INFORMATION
- PEDMARK is contraindicated in patients with history of a severe hypersensitivity to sodium thiosulfate or any of its components.
- Hypersensitivity reactions occurred in 8% to 13% of patients in clinical trials. Monitor patients for hypersensitivity reactions. Immediately discontinue PEDMARK and institute appropriate care if a hypersensitivity reaction occurs. Administer antihistamines or glucocorticoids (if appropriate) before each subsequent administration of PEDMARK. PEDMARK may contain sodium sulfite; patients with sulfite sensitivity may have hypersensitivity reactions, including anaphylactic symptoms and life-threatening or severe asthma episodes. Sulfite sensitivity is seen more frequently in people with asthma.
- PEDMARK is not indicated for use in pediatric patients less than 1 month of age due to the increased risk of hypernatremia or in pediatric patients with metastatic cancers.
- Hypernatremia occurred in 12% to 26% of patients in clinical trials, including a single Grade 3 case. Hypokalemia occurred in 15% to 27% of patients in clinical trials, with Grade 3 or 4 occurring in 9% to 27% of patients. Monitor serum sodium and potassium at baseline and as clinically indicated. Withhold PEDMARK in patients with baseline serum sodium greater than 145 mmol/L.
- Monitor for signs and symptoms of hypernatremia and hypokalemia more closely if the glomerular filtration rate (GFR) falls below 60 mL/min/1.73 m2.
- Administer antiemetics prior to each PEDMARK administration. Provide additional antiemetics and supportive care as appropriate.
- The most common adverse reactions (≥25% with difference between arms of >5% compared to cisplatin alone) in SIOPEL 6 were vomiting, nausea, decreased hemoglobin, and hypernatremia. The most common adverse reaction (≥25% with difference between arms of >5% compared to cisplatin alone) in COG ACCL0431 was hypokalemia.
Please see full Prescribing Information for PEDMARK.
INDICATIONS AND USAGE
PEDMARK (sodium thiosulfate injection) is indicated to reduce the risk of ototoxicity associated with cisplatin in pediatric patients 1 month of age and older with localized, non‑metastatic solid tumors.
Limitations of Use
The safety and efficacy of PEDMARK have not been established when administered following cisplatin infusions longer than 6 hours. PEDMARK may not reduce the risk of ototoxicity when administered following longer cisplatin infusions, because irreversible ototoxicity may have already occurred.